Abstract
BACKGROUND
Few Tx options are available for pts with inadequately controlled PV. European LeukemiaNet defined resistance/intolerance was seen in ≈25% pts treated with HU (Alvarez-Larran et al, 2012). In the HU-resistant/intolerant PV pts evaluated in the pivotal RESPONSE study (week [wk] 208), RUX was well tolerated and superior to standard therapy in achieving durable hematocrit (HCT) control, hematologic response, and spleen size and symptom reductions. This Ph 3b ETP study was planned to provide RUX Tx to HU-resistant/intolerant PV pts, who have no alternative standard Tx, and are not eligible for any ongoing clinical studies. Results from wk 24 data cutoff of this study (Devos et al) were presented at ASH 2017. Here, we report consolidated findings from the ETP study at wk 96 data cutoff (Dec 29, 2017 [final database lock]) to further support the use of RUX in this pt population with an unmet medical need.
METHODS
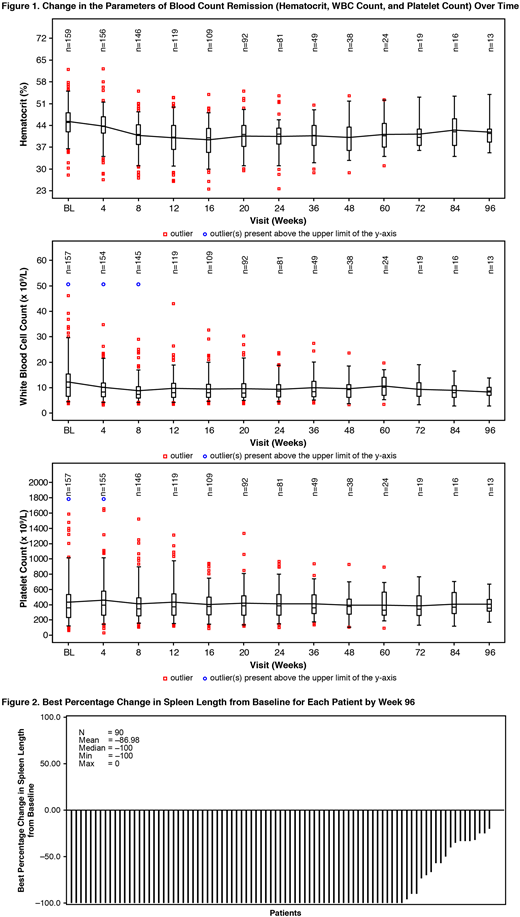
RUX Tx was initiated at a starting dose of 10 mg bid (could be titrated to a maximum of 25 mg bid). Visits were scheduled every 4 wks until wk 24 and every 12 wks thereafter; final analysis was done when all pts had been followed for 30 days after discontinuation of Tx or completion of Tx per protocol (transitioned to commercial RUX or until Dec 31, 2017, whichever date occurred first). The primary endpoint was to assess the safety of RUX. Secondary endpoints included change in HCT level, change in spleen length, and pt-reported outcomes (change in MPN-SAF TSS score). HCT control at wk 24 was defined by absence of phlebotomy (PBT) eligibility starting at wk 8 and continuing through wk 24, with no more than 1 PBT eligibility occurring after first dose date and prior to wk 8. PBT eligibility was defined by confirmed HCT >45% (at least 3 percentage points higher than HCT at baseline [BL]), or confirmed HCT >48%. Blood count remission at wk 24 was defined by HCT control, and white blood cell count <10 × 109/L, and platelet count ≤400 × 109/L.
RESULTS
At data cutoff, 161 pts with PV were enrolled (BL characteristics similar to that presented at ASH 2017). End of Tx was reported for all 161 pts: Tx duration completed per protocol (141 pts), adverse event (AE [12 pts]), consent withdrawal (3 pts), pt decision (2 pts), disease progression (2 pts), and death (due to accident [1 pt]). The median exposure was 25.1 wks (range, 0.4-104.7), and median dose intensity of RUX was 20.0 mg/day (range, 6.7-47.7). AEs (regardless of study drug relationship) led to dose adjustment/interruption in 37.9% pts and study drug discontinuation in 8.7% pts. The most common hematologic AEs (rate=number of events per 100 pt-year exposure [pt-year exposure=110.2]; all grades]) included anemia (31.8) and thrombocytosis (10.0), while headache (24.5), diarrhea (14.5), constipation (12.7), and fatigue (12.7) were the most frequent non-hematologic AEs. For all reported grade 3/4 AEs, exposure-adjusted rate was less than 3. Thromboembolic events (all grade; Standardized MedDRA Query) were reported in 3 pts. Disease progression was reported in 4 pts (myelofibrosis=3 pts; acute myeloid leukemia=1 pt). The incidence of other neoplasms (regardless of study drug relationship) was low (leiomyoma, malignant melanoma, marginal zone lymphoma, renal cancer [1 pt each]; squamous cell carcinoma [2 pts]; basal cell carcinoma [3 pts]). Infections (all grades) were reported in 57 pts (grade 3/4 in 5 pts).
At wk 24, 73 pts (45.3% [95% CI, 37.5%-53.4%]) achieved HCT control; hematologic remission was seen in 29 pts (18% [95% CI 12.4%-24.8%]). Changes in blood count parameters over time are shown in Fig. 1. In evaluable pts (N=105), use of PBT decreased from BL (39 PBTs between screening and BL) to end of Tx (5 PBTs in 12 wks prior). Best spleen response from BL for each pt by wk 96 is shown in Fig. 2. At least 50% spleen length reduction was seen in 86.7% (78/90) of pts from BL at any time in the study. Overall, 33.8% (46/136) of pts had ≥50% reduction in MPN-SAF TSS from BL at the end of Tx.
CONCLUSION
The observed safety profile of RUX in the ETP study was consistent with that of the RESPONSE studies. Efficacy results were close to the observed values in the RESPONSE studies. RUX Tx resulted in HCT control, hematologic remission, spleen response, and symptom reduction in this HU-resistant/intolerant pt population in need of a viable Tx option. Safety and efficacy findings from this ETP study support the use of RUX for pts with inadequately controlled PV, an unmet medical need.
Foltz:Novartis: Consultancy, Honoraria, Research Funding; Incyte: Research Funding; Promedior: Research Funding; Gilead: Research Funding. Leber:Novartis Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis Canada: Honoraria, Membership on an entity's Board of Directors or advisory committees. de Almeida:Celgene: Speakers Bureau; Novartis: Speakers Bureau. Ranta:Novartis: Consultancy. Cartes:Novartis: Honoraria. Kiladjian:Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP Orphan: Membership on an entity's Board of Directors or advisory committees, Research Funding. Chrit:Novartis: Employment, Equity Ownership. Yin:Novartis: Employment. Morando:Novartis: Employment, Equity Ownership. Devos:Celgene: Consultancy; Novartis: Consultancy; Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal